Latest NCERT Solutions 2024: Class 6 science chapter 3 Separation of Substances notes in English | कक्षा 6 विज्ञान अध्याय 3 एनसीईआरटी समाधान 2024: पदार्थों का पृथक्करण | कक्षा 6 विज्ञान के प्रश्न उत्तर पाठ 3 | Class 6 Science Chapter 3 question answer in English | कक्षा 6 विज्ञान पाठ 3 प्रश्न उत्तर | कक्षा 6 विज्ञान पाठ 3 पदार्थों का पृथक्करण | कक्षा 6 विज्ञान पाठ 3 पदार्थों का पृथक्करण Question answer | कक्षा 6 विज्ञान पाठ 3 पदार्थों का पृथक्करण Question answer | कक्षा 6 विज्ञान पाठ 3 प्रश्न उत्तर | पदार्थों का पृथक्करण Class 6 PDF | Class 6 Science Chapter 3 question answer in English | पदार्थों का पृथक्करण Class 6 Notes | कक्षा 6 विज्ञान अध्याय 3 | पदार्थों का पृथक्करण Class 6 Notes |
Separation of Substances
In nature, substances are often found in the impure state or mixed with other substances. A pure substance is made of only one type of atoms or molecules. Its components cannot be further separated by physical methods.
A mixture is an impure substance which contains different types of molecules (pure substances). It is a physically formed blend of two or more dissimilar substances.
Need for Separation of Mixtures
- To separate two or more substances, each being useful to us.
- Examples: Rice and flour, sand and pebbles, kerosene and petrol, salt and iodine
- To separate useful substances from non-useful ones Examples: Sand and water, dirt and clothes, iron and garbage.
- To separate impure or harmful substances from useful ones Examples: Stone and rice, pesticides and water, weeds and crops.
कक्षा 6 विज्ञान अध्याय 3 एनसीईआरटी समाधान 2024: पदार्थों का पृथक्करण
Pure Substance
Pure substances are those substances that are made up of only one kind of particles. More precisely, they are composed of only one type of atoms or molecules.
There are two types of pure substances:
Element: They consist of linking one or more same atoms. For example, hydrogen atoms, oxygen atoms and iron, etc.
Compound: When different atoms or molecules are linked together, then they form a compound. For example, carbon dioxide, sodium chloride and water, etc.
Adulteration
When unwanted components are added to the food items then they are called adulteration, like small stones in rice.
कक्षा 6 विज्ञान अध्याय 3 एनसीईआरटी समाधान 2024: पदार्थों का पृथक्करण
Mixture
The formation of any substance by mixing one or more substances is called a mixture. Moreover, the mixed substances possess unique property. Some examples are:-
- Milk is composed of a mixture of water and cream.
- Water, carbon dioxide and sugar are mixed to form an aerated drink
- The mixture of water, dead organic matter and broken rocks, and minerals create soil.
- The mixture contains different substances called its components that can be present in ratios.
कक्षा 6 विज्ञान अध्याय 3 एनसीईआरटी समाधान 2024: पदार्थों का पृथक्करण
Properties of Mixtures:
- The components ratio in a mixture cannot be fixed.
- The components can be separated by simple methods of separation. For example, separating stones from rice by hand.
- The melting and boiling point of mixtures is not fixed.
Types of Mixtures:
There are two types of mixtures:
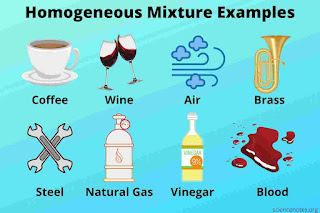
Homogeneous mixture: In a mixture, the components are evenly distributed. Some examples of homogeneous mixture are the dissolution of salt in the water, alloys(made up of copper steel and bronze), and pure air.
कक्षा 6 विज्ञान अध्याय 3 एनसीईआरटी समाधान 2024: पदार्थों का पृथक्करण
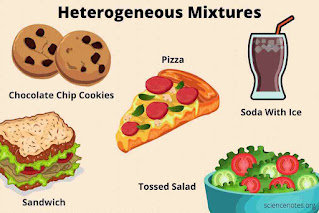
Heterogeneous mixture: These mixtures do not have evenly distributed components. Some examples are fruit salad and chocolate chip cookies, etc.
Separation Of Substances from Mixture
The following purposes for separating components from mixtures are:
- To remove impurities or harmful components: The impurities affect health. Therefore, they need to be removed. For example, purifying river water for drinking, removing stones and other impurities from rice and pulses.
- To obtain useful components: The distillation of petroleum to obtain useful components like petrol, kerosene, diesel and white petroleum jelly.
- To obtain pure components: The separation of pure metals from their natural forms is called ores.
कक्षा 6 विज्ञान अध्याय 3 एनसीईआरटी समाधान 2024: पदार्थों का पृथक्करण
Methods of separation
1. Separation is a method of separating one substance from a mixture of two or more substances,
2. Separation of Solids from Other Solids.
3. Separation solids from the mixture of solids can be performed by the following methods:
Different Methods of Separation
Handpicking: It is a method of separation by using hands. The following purposes where handpicking is applicable are:
- When components are visible to the naked eye.
- The shape, size and colour of the components are different from the useful materials.
- The size of the components is large
Some examples of handpicking methods are small stones, broken grains are separated from rice, wheat and pulses.
कक्षा 6 विज्ञान अध्याय 3 एनसीईआरटी समाधान 2024: पदार्थों का पृथक्करण
Threshing:
The process of separating grains from the harvested stalk is called threshing. It can be performed by the following ways:
- Beating the dry stalks on the hard surface by hands.
- Threshing machines are also used to separate dried grains.
- Crushing the stalks by animals like bullocks.
For example, threshing is used to separate the grains from the stalks of harvested rice and wheat crops.
कक्षा 6 विज्ञान अध्याय 3 एनसीईआरटी समाधान 2024: पदार्थों का पृथक्करण
Winnowing:
The process of separating grains from the husk is called winnowing in this method, the mixture of grains and husk drops from a height, then the wind carried the husk away from the grains, as grains are heavier than the husk, they form a heap near the performer. Some examples of winnowing method are separation of sand from the powdered dry leaves.
कक्षा 6 विज्ञान अध्याय 3 एनसीईआरटी समाधान 2024: पदार्थों का पृथक्करण
Sieving:
The process of separating the components according to their size is called sieving. A sieve is composed of a net or mesh. The size of the pores on the net or mesh depends on the size of the wanted materials.
The most common example of sieving method is the separation of bran from the wheat flour.
कक्षा 6 विज्ञान अध्याय 3 एनसीईआरटी समाधान 2024: पदार्थों का पृथक्करण
Magnetic Separation:
The process of separating magnetic properties possessing materials from substances is called magnetic separation. In a mixture, one component is attracted to the magnet while the other one is not. For example, the separation of iron from the sand by a magnet.
Separation of Solids from Liquids
- In this separation, the method is dependent on the solubility of the substance.
- Separating insoluble solids from liquids
- Such particles that are not soluble in the water can be separated by the following methods:
Sedimentation and Decantation:
Sedimentation is the process of separating insoluble materials from liquid. The insoluble components settle down at the bottom of the liquid forms sediment, and the liquid above the sediment is called supernatant.
Afterwards, the supernatant is carefully removed out of the container without disturbing the sediment is called decantation. For example, separation of fine particles from the muddy water can be done by this method.
कक्षा 6 विज्ञान अध्याय 3 एनसीईआरटी समाधान 2024: पदार्थों का पृथक्करण
Filtration:
The separation of insoluble components from the mixture using a filter is called filtration. The fine particles that are stored on the filter paper or funnel are called a residue, while the pure liquid that passes through the funnel into a container is called the filtrate.
कक्षा 6 विज्ञान अध्याय 3 एनसीईआरटी समाधान 2024: पदार्थों का पृथक्करण
Separating soluble from their solutions
The separation of soluble solids from the mixture as follows:
Evaporation: The process of separating the soluble materials from the solution by heating it is called evaporation. For example, the formation of the common salt from the seawater by evaporation occurs naturally.
Condensation: The process of converting the water vapors into the water by cooling is called condensation. Raining is a good example of this process.
कक्षा 6 विज्ञान अध्याय 3 एनसीईआरटी समाधान 2024: पदार्थों का पृथक्करण
Saturated and Unsaturated Solutions
Saturated Solution: A solution which cannot dissolve more solute at a given temperature is called a saturated solution.
Unsaturated Solution: A solution which can dissolve more solute a given temperature is called an unsaturated. solution.


.jpg)









Please do not enter any spam link in the comment box.